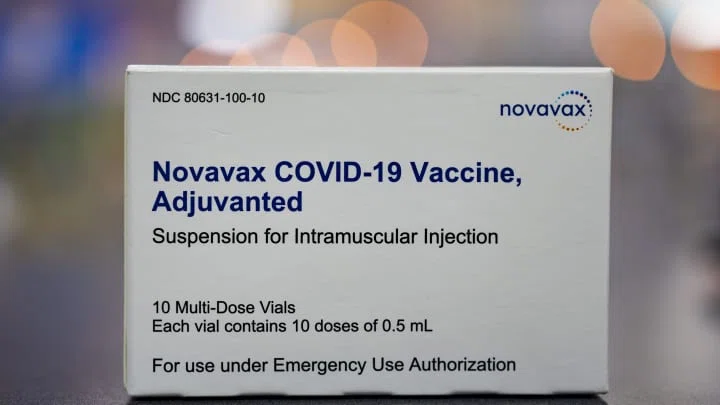
Food and Drug Administration (FDA) of the US has recently approved the COVID-19 vaccine by the biotechnology company ‘Novavax’ for emergency use in teenagers Biotechnology company Novavax announced on Friday that its Covid-19 vaccine has been authorized for emergency use by the U.S Food and Drug Administration for adolescents between the ages of 12 and 17. In July, Novavax’s two-dose Covid-19 vaccine for adults ages 18 and over got its emergency approval from the FDA. Having more vaccine options for adults and children will “hopefully help increase vaccination rates, particularly…
Read MoreWednesday, March 12, 2025
Recent posts